For many compounds, the systematic name and the common name are both used frequently, requiring familiarity with both. For example, the systematic name for NO is nitrogen monoxide, but it is much more commonly called nitric oxide. Similarly, N 2 O is usually called nitrous oxide rather than dinitrogen monoxide.. CH2CH3. Here’s the best way to solve it. The basic rules for naming are- 1. Find the longest C chain which contains the f.. Give the systematic name for each of the following compounds: 1. 2. CH3CH2C (CH3)3 CH3 CH3 CH_CH_CHCH_CCH CH; 3. . CH:CH-C (CH2CH3)2CH2CH2CH3 4. CH3 CH2CHCH,CH,CHCH CH2CH3 .
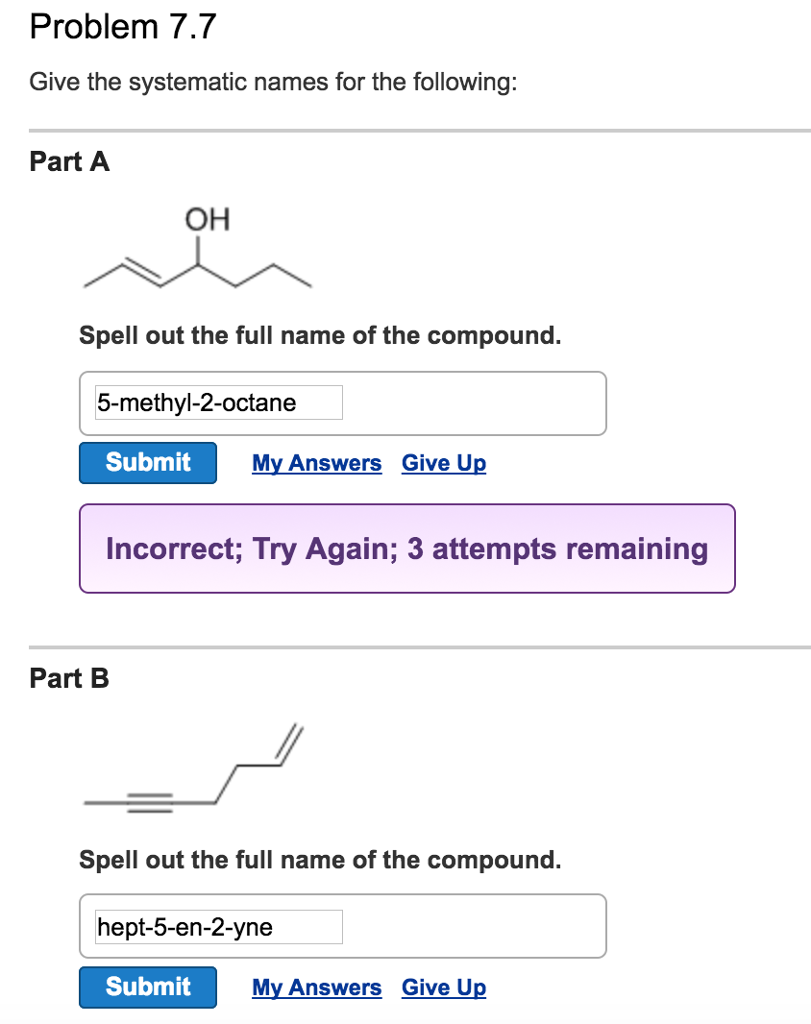
Solved Problem 7.7 Give the systematic names for the
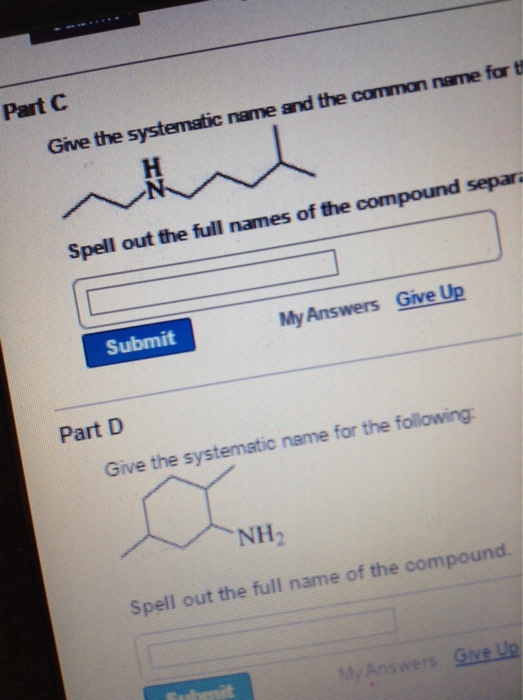
Solved Give the systematic name and the common name for the
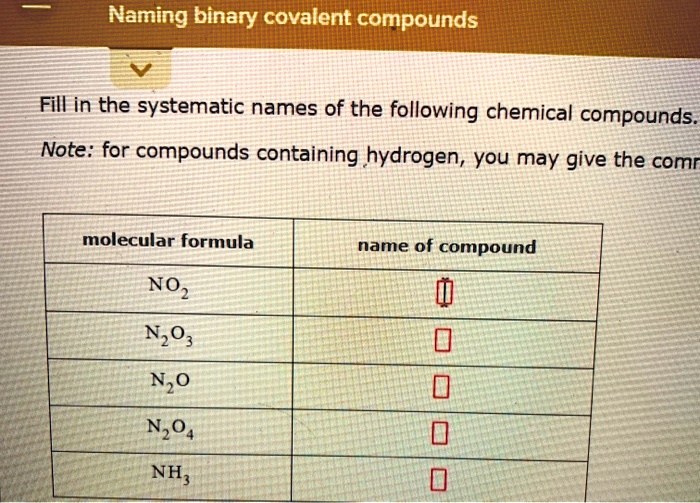
SOLVED Naming binary covalent compounds Fill in the systematic names of the following chemical
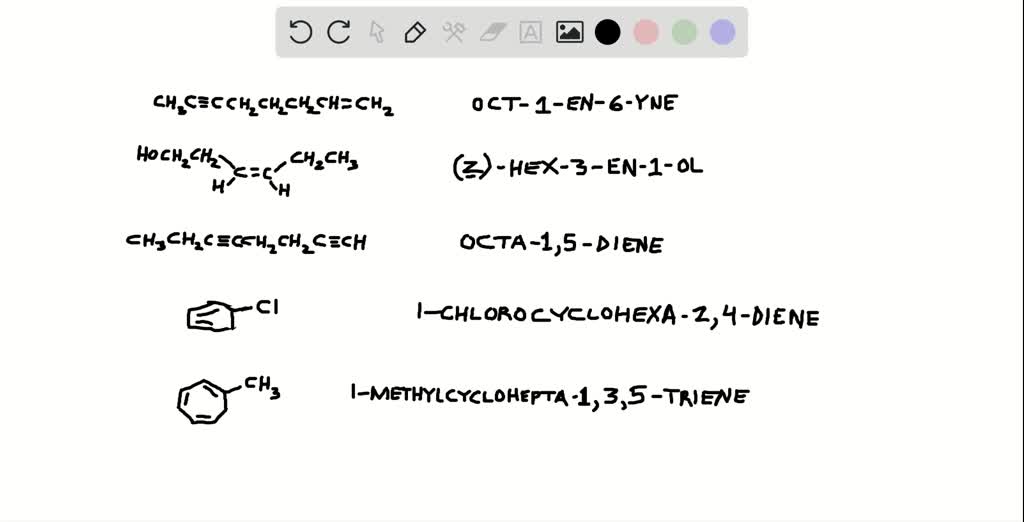
SOLVEDGive the systematic name for each of the following compounds
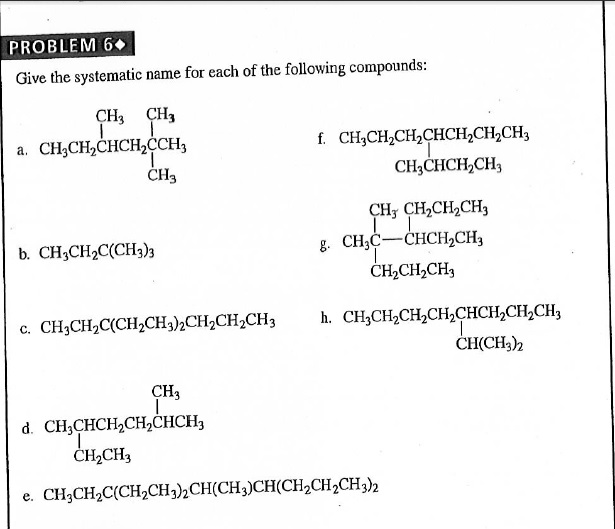
Solved Give The Systematic Name For Each Of The Following…
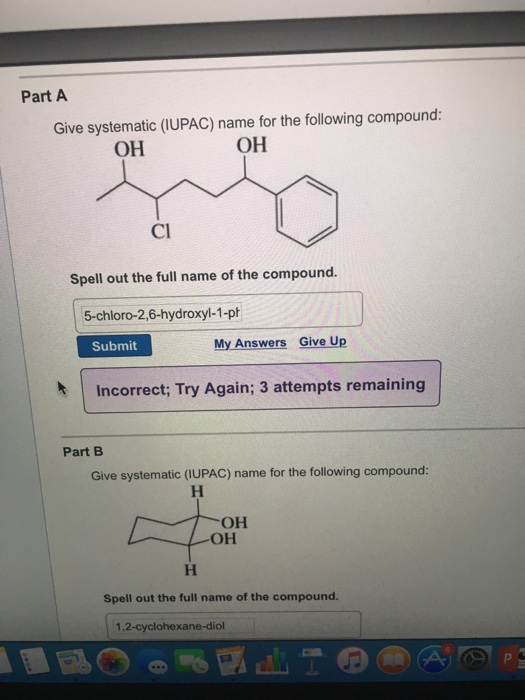
Solved Give systematic (IUPAC) name for the following
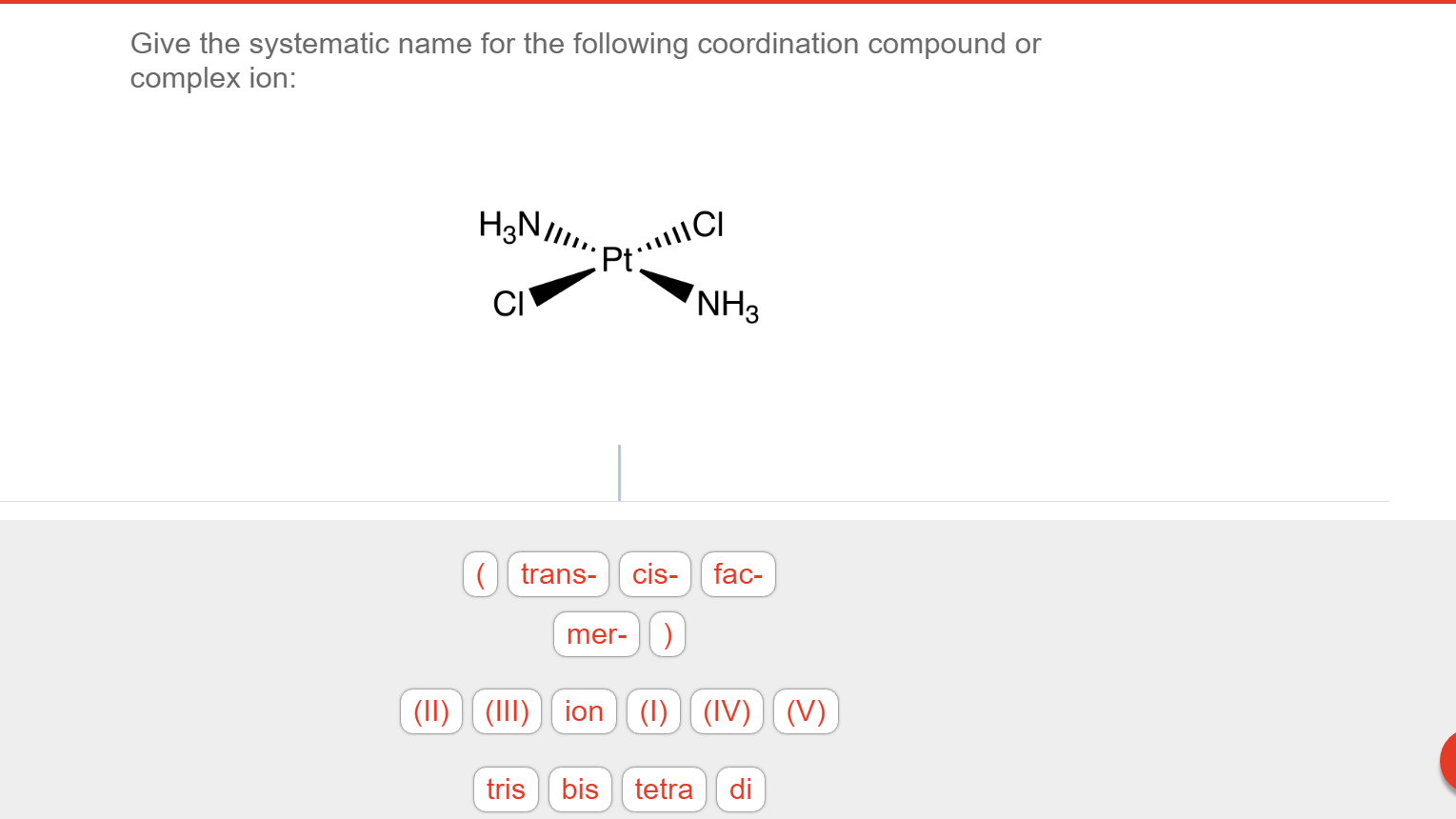
Solved Give the systematic name for the following
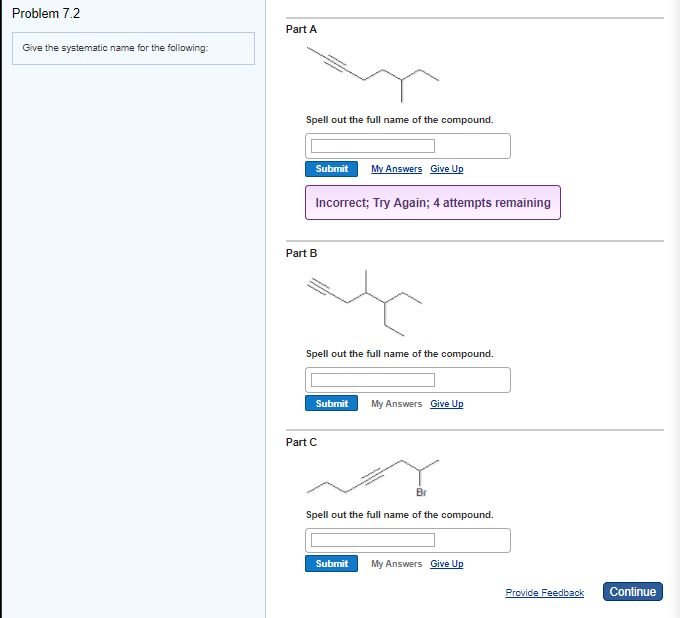
Solved Problem 7.2 Part A Give the systematic name for the
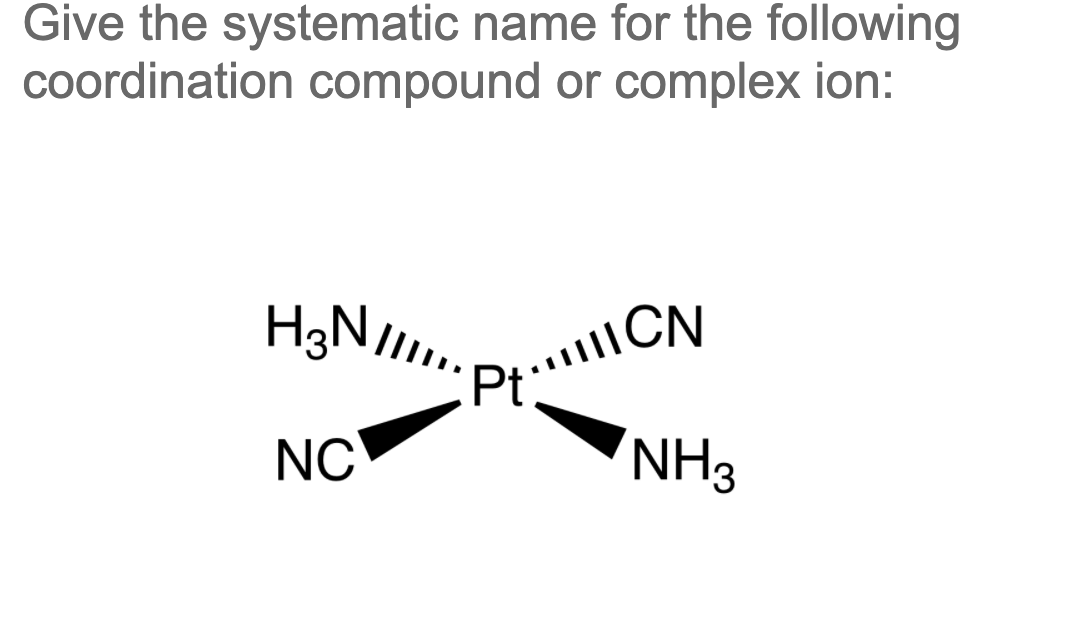
Solved Give the systematic name for the following
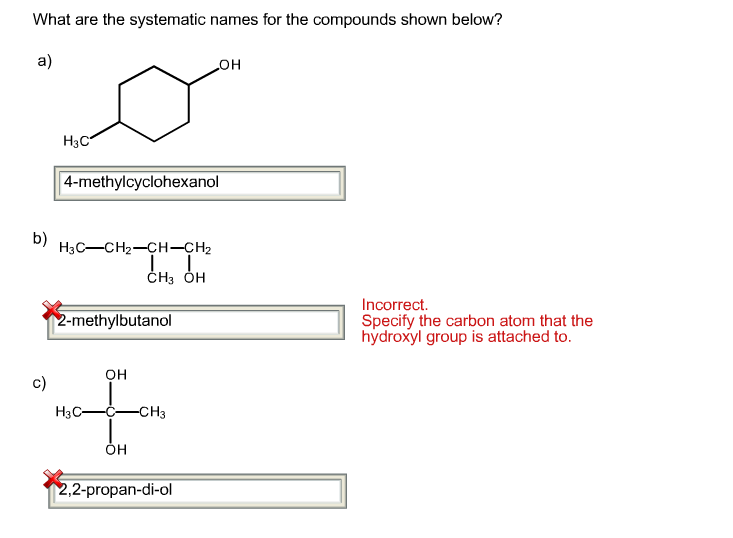
Solved What are the systematic names for the compounds shown
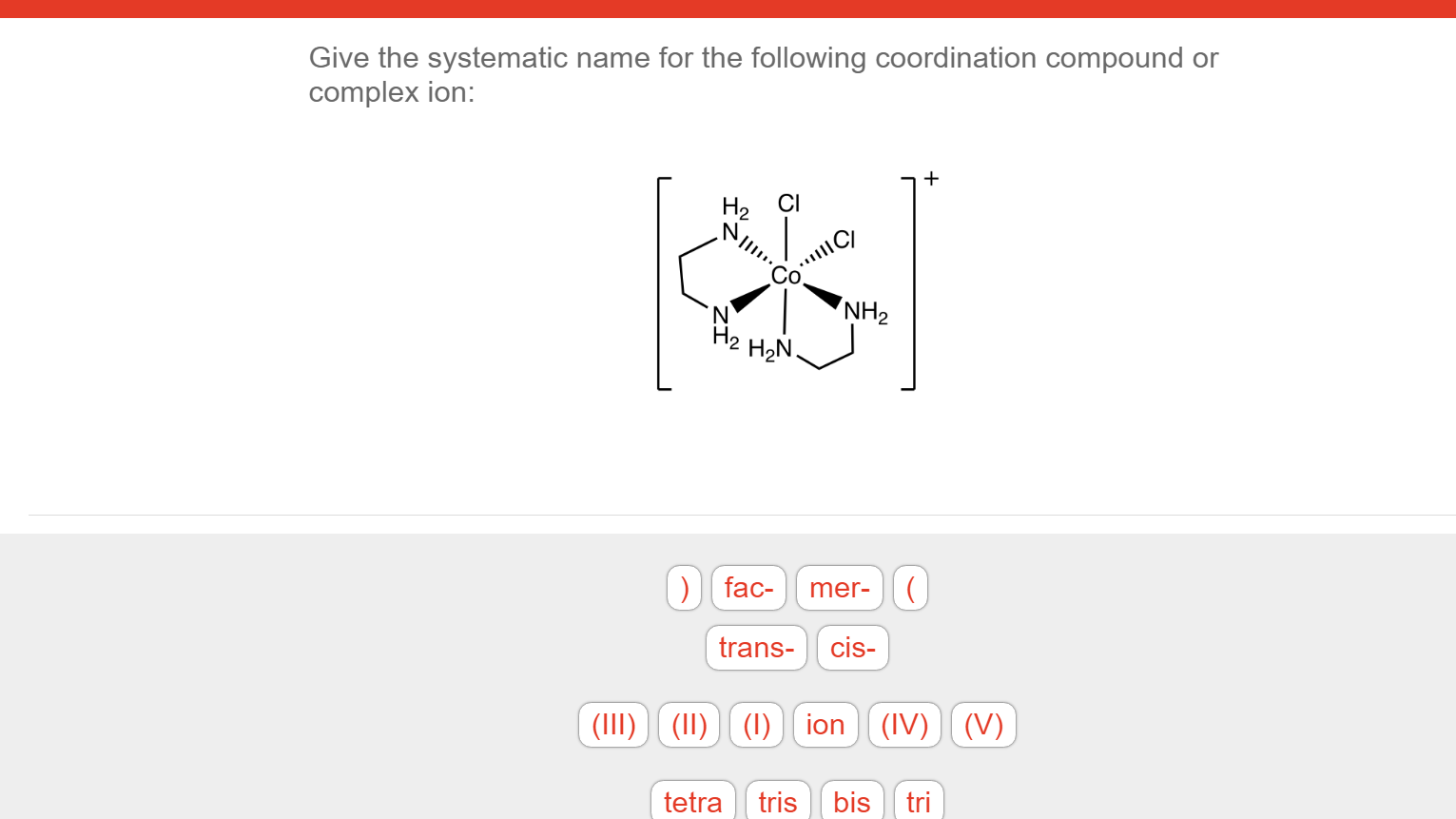
Solved Give the systematic name for the following

Solved Give systematic names for the following compounds
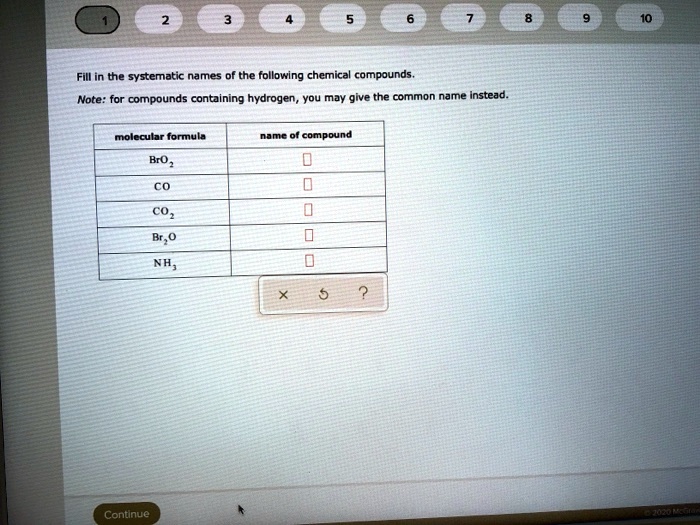
SOLVED Fill in the systematic names of the following chemical compounds. Note for compounds
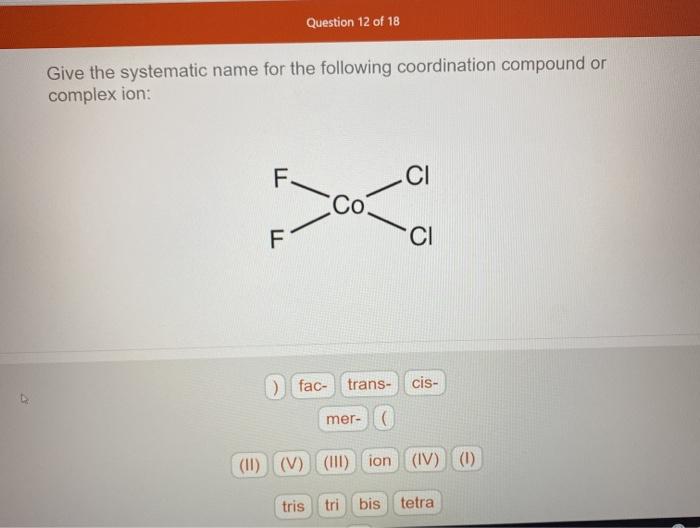
Solved Question 12 of 18 Give the systematic name for the
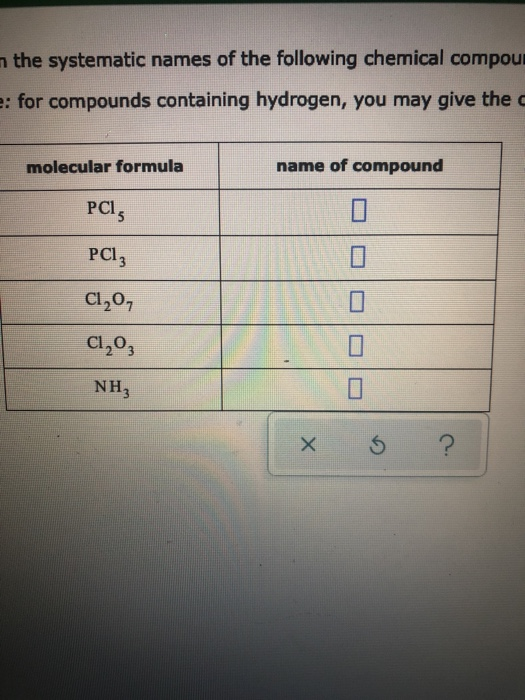
Solved the systematic names of the following chemical
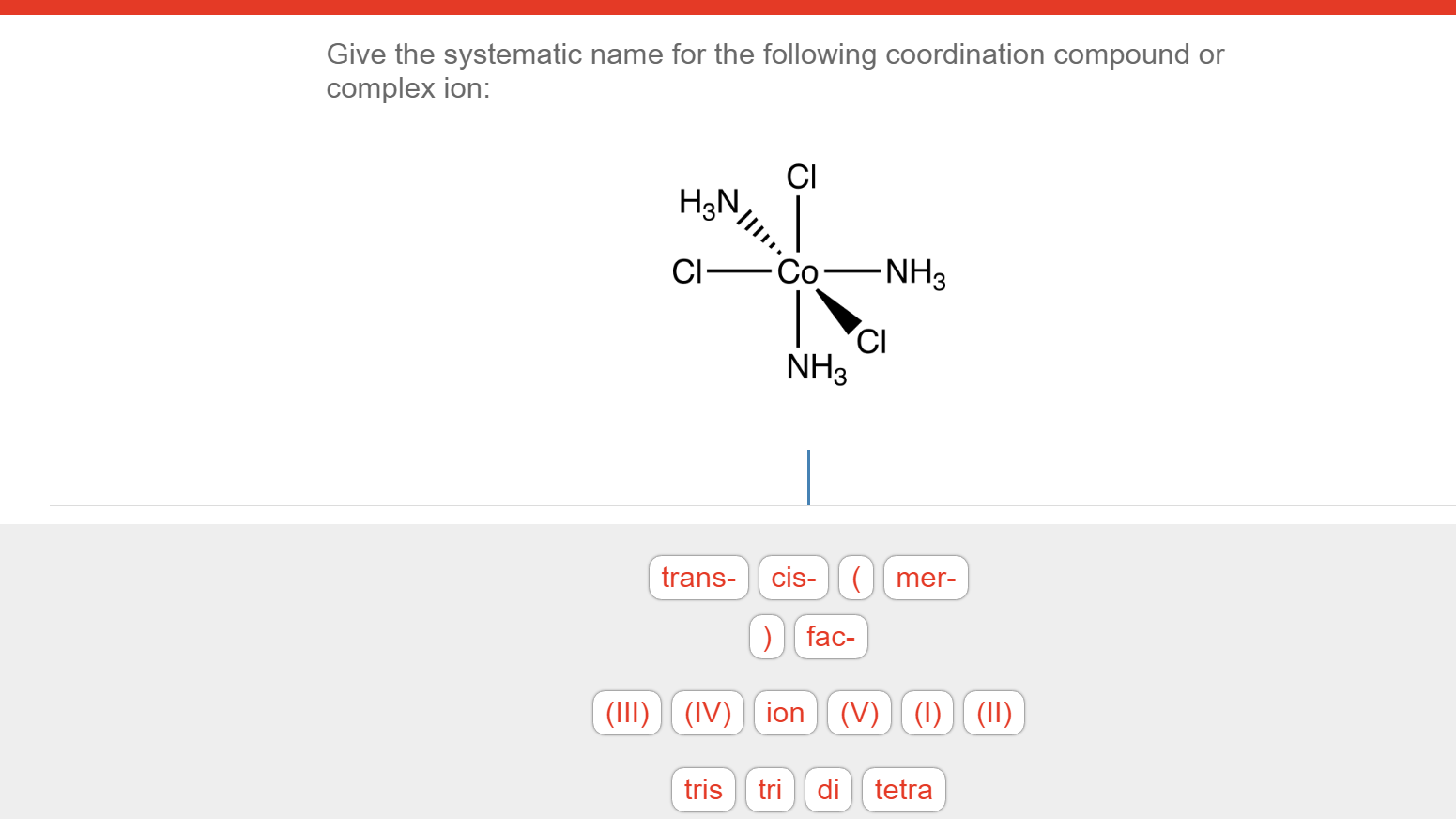
Solved Give the systematic name for the following
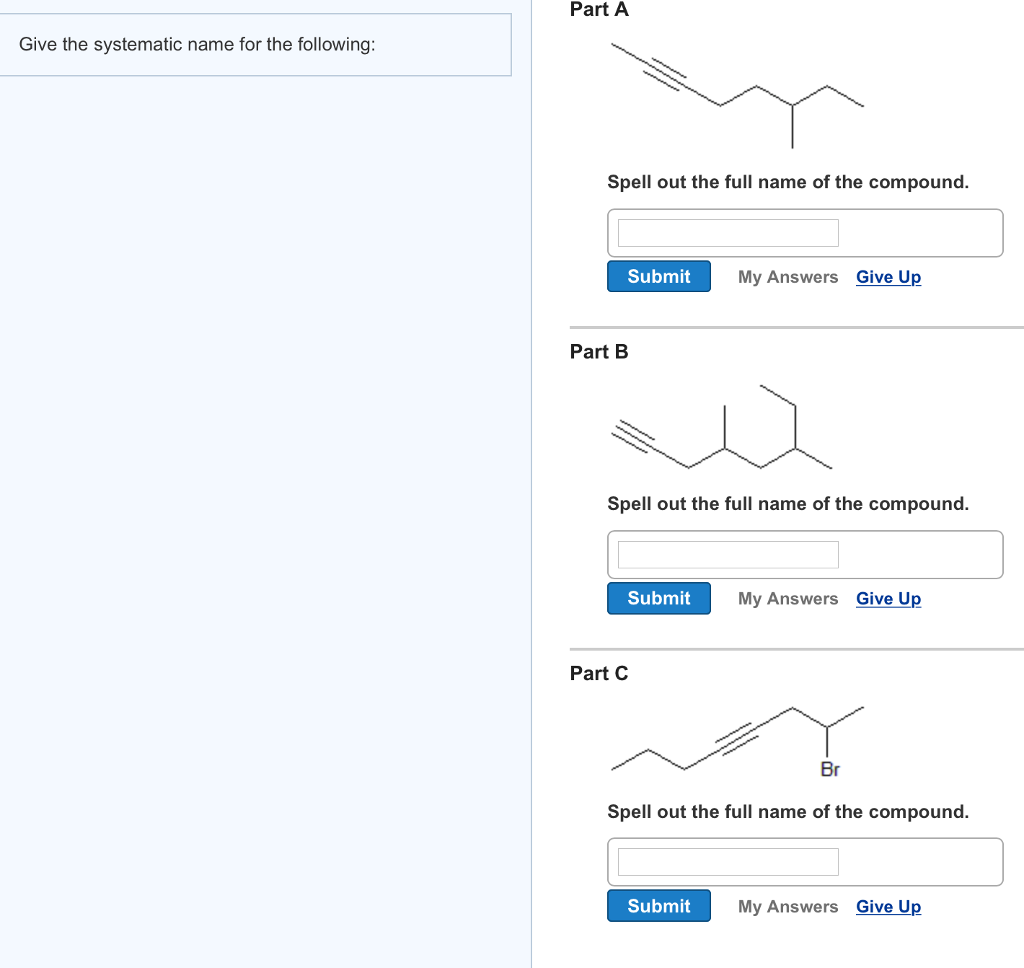
Solved Part A Give the systematic name for the following
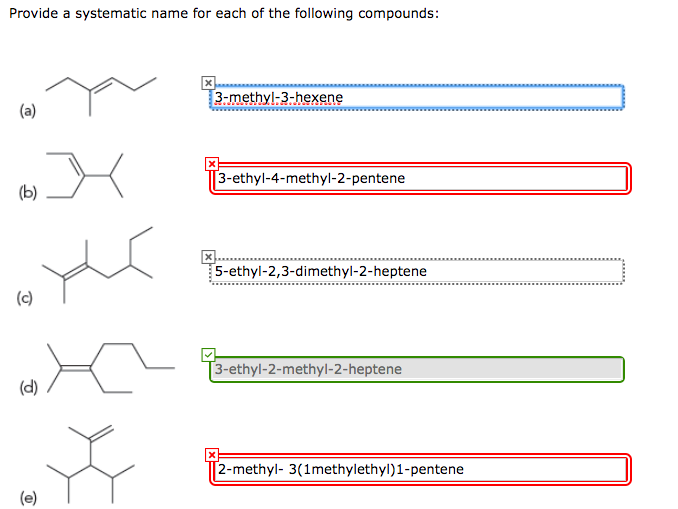
Write the Systematic Names for the Given Compounds
[Solved] Provide a systematic name for the following compound…. Course Hero

Solved Give systematic names for the following compounds
Identify the formula and name of the molecule. Study with Quizlet and memorize flashcards containing terms like Identify the elements that are diatomic., Give the systematic name of each covalent compound. Spelling counts. SF4 P4S3 CO P3N5, Determine the formula unit for the compound formed when each pairs of ions interact. and more.. IUPAC Rules. In order to name organic compounds you must first memorize a few basic names. These names are listed within the discussion of naming alkanes. In general, the base part of the name reflects the number of carbons in what you have assigned to be the parent chain. The suffix of the name reflects the type (s) of functional group (s.